Abbott Laboratories [Abbott Park / United States of America]
Abbott Laboratories [Abbott Park / United States of America] located in ABBOTT PARK IL, United States of America under the [Abbott Laboratories] organization had its last known inspection on 04 Oct 2019. There are 46 known inspections on record.
Site Details
Industries:
Human Drugs,
Biologics,
Medical Devices,
Animal Health
Tags:
Human Drugs,
FDF Manufacturer,
Biologics,
Medical Devices,
Medical Device: In Vitro Diagnostics,
Intermediates Manufacturer,
Non-Clinical Lab (GLP),
Animal Health,
Animal Health: Animal Drugs,
Corporate Headquarters
Parent Organization:
Abbott Laboratories
Last Inspection Date:
04 Oct 2019
FEI:
1415939
Redica ID:
Investigators:
Debra I Love,
Omotunde O Osunsanmi,
Margaret Torres Vazquez,
Nicholas F Lyons,
Nicole J Clausen,
Nicole S Williams,
Russell K Riley,
Chiang Syin, PhD,
Nicole K Trudel,
Carrie Ann Plucinski,
Rafael Padilla,
Tamara Brey,
Kathleen B Swat,
Jeanne M Morris,
Amanda Dinaro,
Jan B Welch,
Kimberly Lewandowski Walker,
Humera T Khan,
Debra L Boyd Seale,
Debra Boyd Seale,
Anna Flynn,
Lorelei S Jarrell,
Harperd,
Julie D Bringger,
Elliot P Cowam, PhD,
Jan Welch,
Daniel Kearns,
Karen Emaslwey Joseph,
Margaret Mtorres Vazquez,
Maureen C Melvin,
Sarah B Tanksley,
Karen Emasley Joseph,
Lagurta M Mayher,
Stephen D Eich,
James W Plucinski,
Norman Wong,
Chad E Schmear,
Susan A Zullo, PhD,
Gene D Arcy,
Martha T Olone,
Jason F Chancey,
Amanda S Zorn,
Robert L Stevenson,
Lakisha M Williams,
Humera Khan,
Bruce H Mccullough,
Daniel C Kearns,
Prabhu P Raju,
Michael D Robinson,
Joseph A Morkunas,
Laurie A Haxel,
Lisa Hornback,
Roberta W Cunningham,
Eric M Mueller, PharmD,
Dr. Guang Gao, PhD,
Kujtim Sadiku,
Joan A Loreng,
Brian D Nicholson,
Lequita M Mayhew,
Helen B Ricalde,
Todd M Stankewicz,
Susan M Jackson,
Jesse A Vazquez,
Alicia M Mozzachio
Abbott Laboratories [Abbott Park / United States of America]'s Most Recent Documents
| Publish Date | Document Type | Title |
|---|---|---|
| March, 2019 | FDA 483 | Abbott Laboratories - Form 483, 2019-03-29 |
| August, 2015 | EIR | Abbott Laboratories - EIR, 2015-08-11 |
| August, 2015 | FDA 483 | Abbott Laboratories - Form 483, 2015-08-11 |
| August, 2013 | FDA 483 Response | Abbott Laboratories - Form 483R, 2013-09-06 |
| August, 2013 | EIR | Abbott Laboratories - EIR, 2013-08-15 |
Experience Redica Systems’s NEW site dashboards and profiles
Redica Systems customers can leverage the full Quality and Regulatory Intelligence suite, with powerful analysis covering thousands of sites around the world. You can search by FEI, or more powerfully, by the Redica ID, our universal identifier for global life sciences manufacturing sites, an industry first.
Investigator Profiles are not sold as on-demand documents. If you’d like to see one, fill out the form below to connect with our Sales Team and get it in your inbox as little as 24 hours.
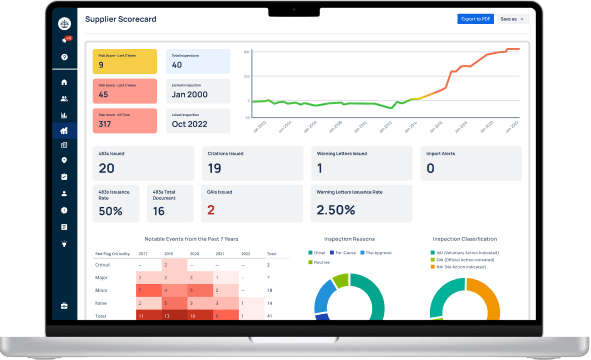
Loading...
Site Profiles Are Just the Tip of the Iceberg
Becoming a Redica Systems customer not only provides you with unlimited access to our extensive inventory of site profiles, but also grants you access to:
Inspection Intelligence
- Pre-Approval Inspection (PAI) trends
- The ability to filter nearly any FDA enforcement action by GxP labels like GMP, GCP, etc.
- Latest 483s: see what the trends are across all FDA Investigators
- CFR Heatmap: Citations aggregated by Subpart by Year
Vendor Intelligence
- Full inspection histories for your vendors down to the specific site level
- All of the functionality mentioned above but isolated to just your vendors
Regulatory Intelligence
- Zero in on the trends for a specific agency/regulator (ex. FDA, MHRA, EMA, Health Canada)
- All the latest "Signals" from agencies around the world, segmented by Country, Type (ex. Guidance, Decree...), Category (ex. Rules/Regulations/Guidance, News, Reports...), Theme (ex. Cell and Gene Therapy, AI...), and more